X chromosome inactivation is a fascinating process that occurs in female cells, allowing them to function properly with two copies of the chromosome while only needing one active copy of its genes. This vital mechanism involves a specialized RNA molecule known as Xist, which plays a crucial role in chromosome silencing and the inactivation of one X chromosome in each cell. Understanding how Xist interacts with chromosomal structures could unlock new treatments for genetic disorders such as Fragile X Syndrome and Rett Syndrome, conditions that stem from mutations on the X chromosome. Recent studies led by researchers like Jeannie T. Lee have illuminated the complex orchestration behind this process, highlighting its potential implications for gene therapy and chromosomal research. As science delves deeper into the mechanisms of X chromosome inactivation, the hope is that such insights could offer relief to thousands affected by these debilitating genetic disorders.
The phenomenon of X chromosome silencing represents a crucial biological strategy in females, enabling them to manage gene expression from their two X chromosomes efficiently. This intricate process, governed by the Xist RNA molecule, ensures that only one X chromosome remains active in each cell, thus avoiding an overabundance of gene products. By harnessing knowledge about this gene regulation mechanism, scientists are exploring innovative therapies for conditions such as Fragile X Syndrome and Rett Syndrome, which are linked to X-linked gene mutations. The ongoing research into chromosome inactivation not only sheds light on fundamental genetic processes but also paves the way for developing potential treatments for various genetic disorders. As we continue to unravel the intricacies of this biological phenomenon, we edge closer to groundbreaking solutions for individuals battling the impacts of X-linked diseases.
Understanding X Chromosome Inactivation
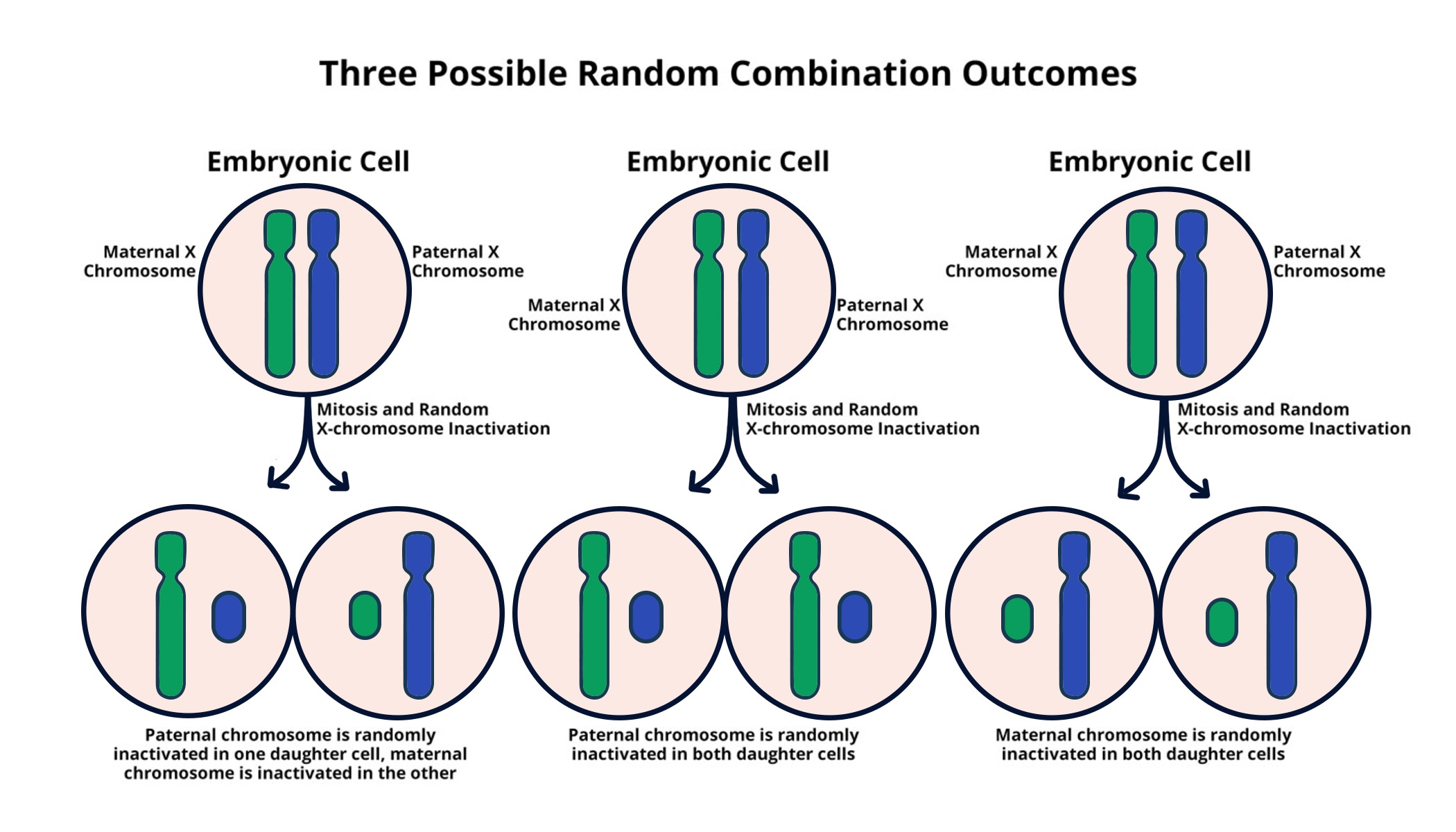
X chromosome inactivation (XCI) is a crucial biological process in female mammals, where one of the two X chromosomes is silenced to balance gene dosage between males and females. This mechanism allows for the prevention of double expression of X-linked genes that could lead to cellular dysfunction. The inactivation process involves intricate molecular interactions, primarily driven by the Xist RNA molecule, which plays a pivotal role in marking and modifying the chromatin structure of the inactivated X chromosome. Understanding this process not only elucidates basic cellular mechanisms but also has profound implications for genetic disorders linked to the X chromosome.
Recent studies have unveiled the fascinating dynamics within the cellular environment during XCI. The gelatinous substance noted by researchers forms an essential part of this silencing process, providing a supportive framework for the mechanisms of gene accessibility and regulation. As Xist interacts with this ‘Jell-O-like’ substance, it alters the properties of the chromatin, promoting regions for accessibility while simultaneously leading to the silencing of non-essential genes. This orchestration of XCI ensures that females can maintain a balanced gene expression profile, crucial for healthy development and function.
Frequently Asked Questions
What is X chromosome inactivation and how does it relate to genetic disorders like Fragile X Syndrome?
X chromosome inactivation is a biological process that occurs in female mammals where one of the two X chromosomes is randomly silenced to ensure equal gene dosage between males and females. This process is crucial because mutations on the X chromosome, such as those causing Fragile X Syndrome, can lead to genetic disorders. In females, while one X chromosome carries the mutation, the other healthy X can often compensate, but in cases where inactivation occurs, the healthy gene may be silenced as well.
How does Xist RNA molecule contribute to X chromosome inactivation?
The Xist RNA molecule plays a central role in X chromosome inactivation by coating and transforming the X chromosome, altering its surrounding environment. Once Xist binds to the X chromosome, it engages with a gelatinous substance (referred to as ‘Jell-O’) around it, leading to changes that facilitate the silencing of genes. This mechanism is essential in ensuring proper chromosome silencing and has implications for diseases linked to X chromosome mutations.
What are the implications of X chromosome inactivation for neurodevelopmental disorders such as Rett Syndrome?
X chromosome inactivation has significant implications for neurodevelopmental disorders like Rett Syndrome, which is caused by mutations in genes on the X chromosome. By understanding the mechanisms of X-inactivation, researchers are exploring ways to potentially unsilence genes that could restore function to areas affected by these mutations, offering hope for therapeutic interventions that could benefit individuals with such disorders.
How does chromosome silencing help in treating diseases linked to the X chromosome?
Chromosome silencing plays a key role in treating X-linked diseases by allowing researchers to target and unsilence the inactivated X chromosome. This could enable the expression of healthy genes that were previously silenced due to X chromosome inactivation. For example, potential therapies for Fragile X Syndrome aim to restore the activity of the healthy gene present on the silenced chromosome, thus addressing the underlying cause of the disorder.
What challenges remain in understanding X chromosome inactivation and its effects on genetic disorders?
Despite advances in understanding X chromosome inactivation, many challenges remain. Researchers are still investigating why restoring function to mutated genes seemingly does not disrupt healthy genes on the X chromosome. Additionally, the complexities of how cells manage gene expression during inactivation require further exploration to develop effective treatments for genetic disorders associated with X-linked mutations.
| Key Concepts | Details |
|---|---|
| X Chromosome Inactivation | Females have two X chromosomes; one must be inactivated to balance gene dosage. |
| Role of Xist | Xist RNA interacts with a gelatinous substance to facilitate inactivation of one X chromosome. |
| Mechanism of Action | Xist alters the properties of the ‘Jell-O’ surrounding the chromosome, enabling inactivation. |
| Therapeutic Implications | Understanding X inactivation opens pathways for treating conditions like Fragile X and Rett syndromes. |
| Research Significance | Jeannie T. Lee’s lab has been pivotal in uncovering the mechanisms of X chromosome inactivation, leading to potential clinical applications. |
Summary
X chromosome inactivation is a crucial biological process that ensures females, with their two X chromosomes, do not express double the amount of genes as males, who have just one. This process involves the complex action of the Xist RNA molecule, which interacts with surrounding chromosomal substances to silence one of the X chromosomes. The ongoing research by Jeannie T. Lee and her team provides exciting prospects for novel treatments for genetic disorders linked to mutations on the X chromosome, such as Fragile X and Rett syndromes. Their findings represent a significant advancement in our understanding of genetics, with the potential to improve the lives of many affected individuals.